Introduction: With rapid treatment advances in multiple myeloma (MM), more patients receive the 3 main classes of MM treatments (immunomodulatory imide drug [IMiD] agents, proteasome inhibitors [PIs], and anti-CD38 monoclonal antibodies [mAbs]) in earlier lines of therapy and become TCE. This is a prerequisite for subsequent treatment with some novel therapies eg, anti-B-cell maturation antigen (BCMA) agents. Published evidence of RW clinical outcomes in patients with TCE MM is scarce in the evolving RRMM treatment landscape. The objective of this study was to assess RW clinical outcomes in patients with TCE RRMM who received treatment post-TCE.
Methods: In this observational study, we analyzed de-identified patient data derived from electronic health records (EHRs) from 2 key United States (US) MM longitudinal databases (Flatiron Health MM [FHMM] and COTA Vantage MM [CVMM]). We identified patients who became TCE between November 2015 and March 2021 and received a subsequent treatment post-TCE; the earliest available end date of the 3rd treatment class was considered the date patients became TCE. The start date of the 1st treatment post-TCE (index treatment) was defined as the index date. We analyzed patient demographics, disease characteristics, prior treatment exposure, refractory status, and index treatments patterns/classes. Clinical endpoints assessed were response rates (overall and complete), overall survival (OS), progression-free survival (PFS), and time to progression (TTP).
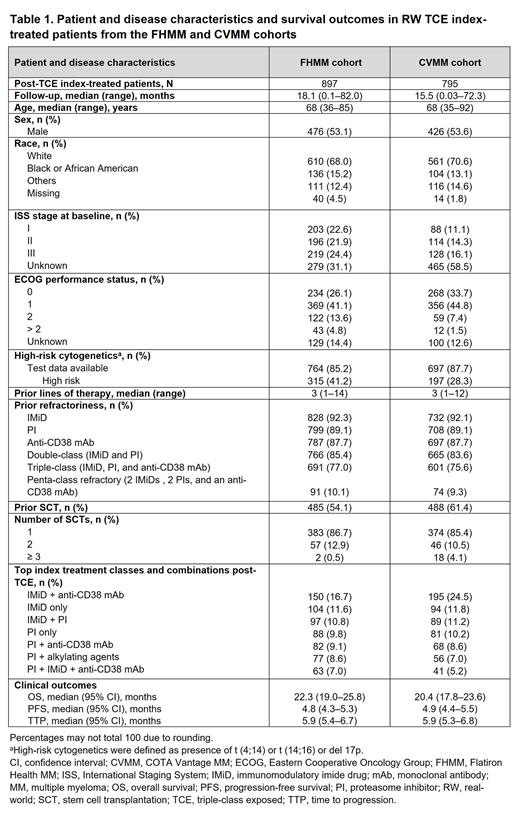
Results: The FHMM and CVMM databases included 1,319 and 1,118 TCE patients, respectively, of whom 897 and 795 received an index treatment post-TCE. Median (range) follow-up for patients who received an index treatment was 18.1 (0.1-82.0) and 15.5 (0.03-72.3) months for FHMM and CVMM cohorts, respectively. Median age for both RW cohorts was 68 years, more than half of patients were men and the majority were White. Patients received a median of 3 lines of prior treatment (range, 1-14 and 1-12 for FHMM and CVMM cohorts, respectively). In both RW cohorts, >75% of patients who received an index treatment were triple-class refractory and approximately 10% were penta-refractory, having received treatment with 2 IMiD agents, 2 PIs, and an anti-CD38 mAb. Approximately 67% and 79% of patients in the FHMM and CVMM cohorts, respectively, had ECOG performance status 0 or 1. Approximately 54% and 61% had received prior stem cell transplantation. IMiD agents either alone or in combination with a PI and/or anti-CD38 mAb constituted the main regimens for index-treated patients. In the CVMM cohort, the overall and complete response rates were 15.2% and 1.7%, respectively; response data were not available in the FHMM cohort. Median OS (22.3 and 20.4 months), median PFS (4.8 and 4.9 months), and median TTP (5.9 and 5.9 months) were comparable for both FHMM and CVMM cohorts.
Conclusions: In this observational cohort study of 2 US RW EHR databases, patients became TCE with a median of 3 prior lines of therapy. Post-TCE, most patients with RRMM still received the same 3 treatment classes with poor response and survival outcomes on their treatments. Despite advances in MM management, this study highlights the need for more efficacious novel treatments for patients with TCE MM and serves as a RW benchmark in the literature for appraising emerging data from novel agents in patients with RRMM.
Disclosures
Lee:Celgene: Consultancy; Janssen: Consultancy, Research Funding; Amgen: Research Funding; Regeneron: Consultancy, Research Funding; Allogene Thereapeutics: Consultancy; Takeda Pharmaceuticals: Consultancy, Research Funding; Monte Rosa Therapeutics: Consultancy; Pfizer: Consultancy; Sanofi: Consultancy; GlaxoSmithKline: Consultancy, Research Funding; Genentech: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding. Jagannath:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria; Takeda: Consultancy; DMC: Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy; Caribou Biosciences: Consultancy; Janssen: Consultancy, Honoraria; Genmab: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy; IMS: Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; ASH: Membership on an entity's Board of Directors or advisory committees; SOHO: Membership on an entity's Board of Directors or advisory committees; Mount Sinai Hospital: Current Employment. Dhanda:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Acheampong:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Patwardhan:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Marshall:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Amin:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Gu:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Ailawadhi:AbbVie, Amgen, Ascentage, BMS, Cellectar, GSK, Janssen, Pharmacyclics, Sanofi: Research Funding; Beigene, BMS, Cellectar, GSK, Janssen, Pfizer, Regeneron, Sanofi, Takeda: Consultancy.